Offer an affordable, accessible, IRB-validated alternative to Spravato for anxiety and depression.

Are your patients struggling with anxiety or depression despite standard treatments?
WonderFlow is an IRB-validated clinical protocol for anxiety and depression, developed by Wonder Research in collaboration with UCLA to deliver safe, effective, outcomes-based care.
By targeting flow state, a measurable neurobiological condition tied to DMN suppression, dopamine modulation, and gamma synchrony, WonderFlow improves mood, resilience, and cognitive flexibility.


- SSRIs: <40% remission, 6–8 week delay, common side effects.
- Spravato: In-clinic monitoring required, $6,000–$8,000/month.
- WonderFlow: 89% symptom reduction in >1,000 patients, at-home supervised care, comprehensive protocol for $199/month.


- Daily practices designed to guide the mind into sustained flow state for healing.
- Daily Organic Supplementation: Functional mushrooms and botanicals designed to support neuroplasticity and balance.
- Wonder Ketamine Lozenges (100–400 mg): Administered 4–8x monthly, acting as a neuromodulatory primer for flow-state induction.
- Clinical Integration: Licensed clinicians and coaches provide monitoring, outcome tracking (PHQ-9, GAD-7), and safety protocols.

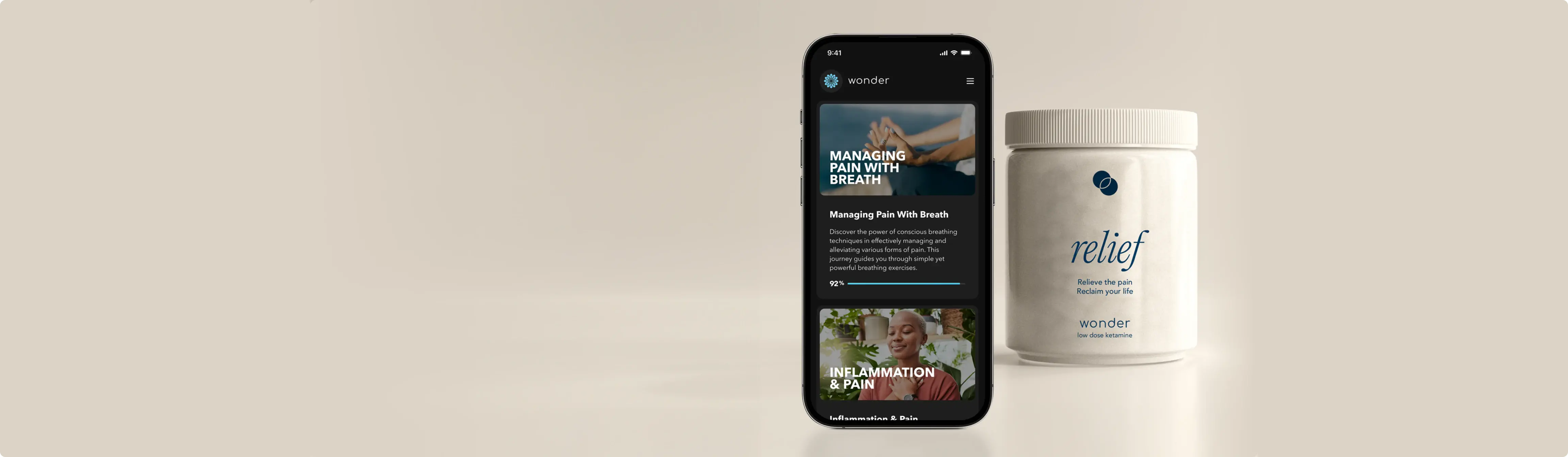
A breakthrough protocol for chronic pain and a safe alternative to opioids.
Wonder Relief is a psychedelic science–based protocol developed by Wonder Research, entering an IRB-cleared clinical trial.
Designed for chronic pain conditions including CRPS, fibromyalgia, neuropathic pain, arthritis, and cancer-related pain, it integrates low-dose ketamine, a proprietary supplement, AI personalization, and behavioral therapy for safe, lasting relief.
- Efficacy – Opioids show diminishing returns and tolerance over time. Ketamine’s NMDA antagonism provides sustained analgesia in CRPS and neuropathic pain.
- Safety – Nearly 75% of U.S. overdose deaths in 2020 involved opioids. Wonder Relief integrates AI misuse detection, clinician oversight, and harm-reduction safeguards.
- Durability – Opioids mask symptoms without retraining pain circuits. Wonder Relief promotes neuroplasticity through ketamine, CBT, and MBSR to recondition maladaptive networks.
- Accessibility & Cost – Chronic opioid therapy drives high costs and ER utilization. Wonder Relief is telemedicine-based, with integrated coaching and peer support for scalable access.

- Pharmacology – Low-dose ketamine lozenges (NMDA blockade, cytokine suppression, GABA potentiation).
- Natural Support – Daily organic supplement with complementary analgesic and calming effects.
- Behavioral Care – CBT, MBSR, and digital self-management modules.
- Clinical & AI Monitoring – WondermedAI tracks adherence, outcomes, and safety; clinicians personalize dosing and interventions.


Pilot Launching 2026
- Validated Psychedelic Protocols – Prescribe WonderFlow, Wonder Relief, and other research-aligned psychedelic-assisted treatments.
- Wonder AI Pharmacology™ – Precision AI and digital-twin modeling to support personalized dosing, monitoring, and care optimization.
- Real-Time Clinical Insights – Track safety, engagement, and outcomes through adaptive analytics.
- Provider Training – CME-accredited education in AI-enabled and psychedelic-assisted care.


mental healthcare excellence